Myasthenia Gravis (MG) is an autoimmune disorder that presents a unique challenge to neurologists due to its varied manifestations and the intricate nature of its diagnosis and treatment. Traditionally understood as a disorder affecting the communication between nerves and muscles, MG leads to muscle weakness and fatigue. However, a closer look reveals that MG can be categorized into two distinct types based on the presence or absence of specific antibodies: Antibody-Positive MG and Seronegative MG. This distinction not only sheds light on the underlying pathophysiology of the disease but also influences the approach to diagnosis and treatment.
How the Brain Moves Voluntary Muscles
Before looking into the different variations of MG, it is important to have a basic understanding of how muscle movement works. The process of moving voluntary muscles is a complex, finely tuned mechanism that involves a series of interactions between the nervous system and the muscular system. Here’s a simplified overview of how the brain controls voluntary muscle movement and how Myasthenia Gravis (MG) disrupts this process.
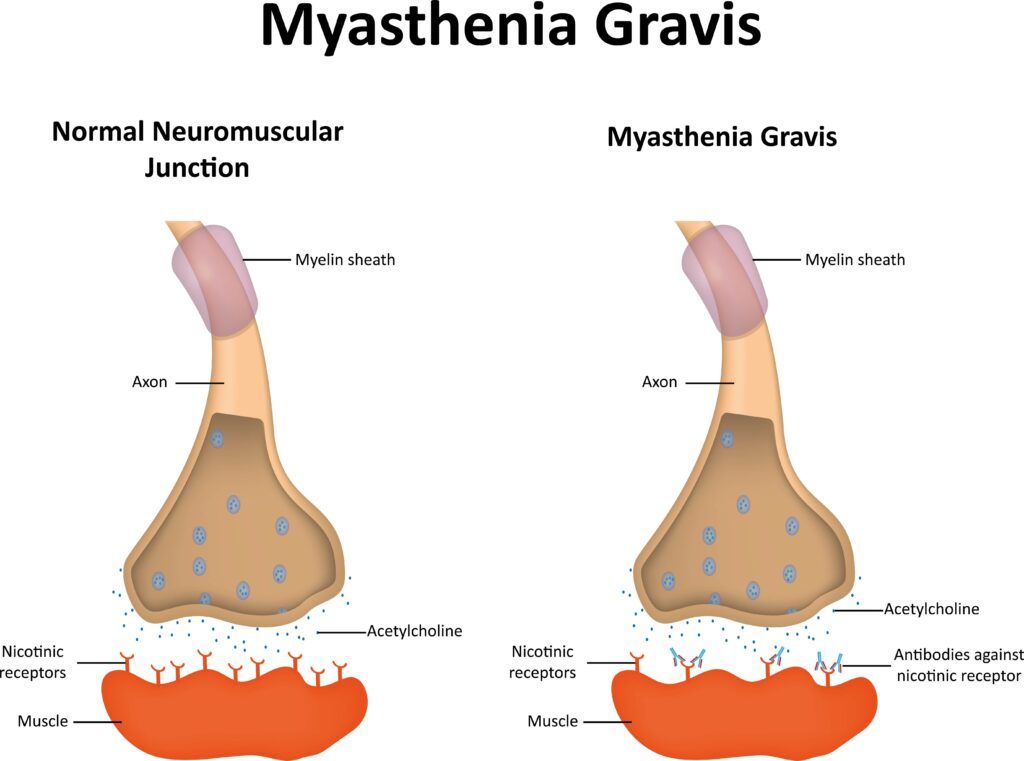
- Initiation in the Brain: The process begins in the brain, where motor neurons in the motor cortex plan and initiate voluntary movements. These neurons send signals through the spinal cord to motor neurons that innervate specific muscles.
- Signal Transmission to Muscles: The signals travel down the motor neurons as electrical impulses. When these impulses reach the neuromuscular junction (NMJ)—the point of communication between the nerve ending and the muscle fiber—they trigger the release of a neurotransmitter called acetylcholine (ACh).
- Muscle Contraction: Acetylcholine diffuses across the synaptic cleft (the gap between the nerve ending and the muscle) and binds to acetylcholine receptors on the muscle fiber’s surface. This binding changes the permeability of the muscle membrane, leading to muscle contraction. After ACh has done its job, it’s broken down by an enzyme called acetylcholinesterase, ensuring that the muscle contraction is a brief, controlled event.
- Feedback to the Brain: Sensory feedback about the movement and the position of the muscle is sent back to the brain, allowing adjustments to be made if necessary. This feedback loop is crucial for the coordination and precision of movements.
Antibody-Positive Myasthenia Gravis
Antibody-Positive MG is characterized by the presence of antibodies that interfere with the normal function of the neuromuscular junction. The most common antibodies associated with MG are those against the acetylcholine receptor (AChR), found in approximately 85% of patients with generalized MG. These antibodies disrupt the normal signaling between nerve endings and muscle fibers, leading to muscle weakness and fatigue. Another less common type involves antibodies against Muscle-Specific Kinase (MuSK), which plays a critical role in the formation and maintenance of the neuromuscular junction. Patients with MuSK antibodies often have a distinct clinical presentation, including muscle weakness that may affect the face, neck, and respiratory muscles more severely.
Seronegative Myasthenia Gravis
Seronegative MG refers to cases where patients exhibit typical symptoms of MG but lack detectable antibodies against AChR or MuSK. This variant accounts for about 10-15% of all MG cases. The term “seronegative” denotes the absence of these specific antibodies in the serum, suggesting a different pathophysiological mechanism. Recent advances have identified antibodies against other components of the neuromuscular junction, such as LRP4 and agrin, in some seronegative patients, indicating that this category may be more heterogeneous than previously thought.
Similarities and Differences
Both Antibody-Positive and Seronegative MG share the hallmark feature of muscle weakness that worsens with activity and improves with rest. The clinical manifestations can vary widely among patients, ranging from ocular symptoms, such as ptosis (drooping eyelids) and diplopia (double vision), to generalized muscle weakness affecting speech, swallowing, and limb movements.
The key difference between these two types lies in their serological profile, which directly impacts the approach to diagnosis and management. Antibody-Positive MG can be diagnosed more straightforwardly through the detection of AChR or MuSK antibodies in the blood, whereas Seronegative MG requires exclusion of other potential causes of the symptoms and may involve more sophisticated diagnostic techniques, such as repetitive nerve stimulation or single-fiber electromyography (EMG), to demonstrate abnormalities consistent with MG.
This table summarizes the key differences and similarities between Seronegative and Antibody-Positive Myasthenia Gravis:
| Feature | Seronegative Myasthenia Gravis (SNMG) | Antibody-Positive Myasthenia Gravis |
| Antibody Profile | No detectable antibodies against AChR or MuSK. | Detectable antibodies against AChR (most common) or MuSK. |
| Pathophysiology | Less understood; may involve other targets like LRP4 and agrin. | Immune system produces antibodies that disrupt neuromuscular transmission by attacking AChR or MuSK. |
| Diagnosis | More challenging due to the absence of AChR and MuSK antibodies. Relies on clinical assessment, response to treatment, and other tests like EMG. | Confirmed by the presence of AChR or MuSK antibodies, alongside clinical symptoms and responses to treatment. |
| Treatment | Similar to antibody-positive MG, including acetylcholinesterase inhibitors, immunosuppressants, plasmapheresis, IVIg, and thymectomy. Response to treatment may vary. | Includes acetylcholinesterase inhibitors, immunosuppressants, plasmapheresis, IVIg, and thymectomy. Specific treatments may be chosen based on antibody profile. |
| Clinical Presentation | Muscle weakness that worsens with exertion. Severity and distribution of muscle weakness can vary. | Muscle weakness that typically worsens with exertion. The presence of specific antibodies can influence the distribution and severity of symptoms. |
Diagnosis
The diagnosis of both antibody-positive and seronegative Myasthenia Gravis (MG) involves a combination of clinical evaluation, serological testing, and neurophysiological studies, but with distinct differences in approach due to the antibody profile. In antibody-positive MG, the diagnosis is significantly aided by the presence of specific antibodies against the acetylcholine receptor (AChR) or muscle-specific kinase (MuSK) in the patient’s blood, serving as a clear biomarker for the condition. The detection of these antibodies through blood tests confirms the diagnosis and helps guide treatment decisions.
On the other hand, seronegative MG presents a diagnostic challenge as it lacks these specific antibodies, necessitating reliance on clinical symptoms, the patient’s response to cholinesterase inhibitors, and additional diagnostic tests such as repetitive nerve stimulation (RNS) and single-fiber electromyography (EMG) to demonstrate abnormalities in neuromuscular transmission. Imaging studies, such as a chest MRI or CT scan, may also be conducted to identify thymoma or other thymic abnormalities. Despite the absence of detectable antibodies, the diagnosis of seronegative MG is reached through a combination of these clinical and electrodiagnostic criteria, highlighting the importance of a comprehensive evaluation in patients presenting with compatible symptoms.
Treatment
The treatment of both antibody-positive and seronegative Myasthenia Gravis (MG) shares many similarities, aimed at improving symptoms and enhancing quality of life, though strategies may be fine-tuned based on the patient’s specific antibody status. For antibody-positive MG, treatments often directly target the pathogenic antibodies or the immune system broadly, using acetylcholinesterase inhibitors like pyridostigmine to improve neuromuscular transmission, immunosuppressive medications to reduce antibody production, and therapies such as plasmapheresis and intravenous immunoglobulin (IVIg) to remove circulating antibodies or modulate the immune response. Thymectomy, the surgical removal of the thymus gland, may also be considered, especially if a thymoma is present or if the patient has generalized AChR-antibody-positive MG.
In contrast, while the same treatments apply to seronegative MG, the approach may be adjusted due to the absence of detectable AChR or MuSK antibodies, with a greater emphasis on symptomatic management and close monitoring of treatment efficacy. The decision-making process in both cases involves a careful consideration of the severity of symptoms, side effects of treatments, and the individual’s overall health, underlining the personalized nature of MG management.
Understanding the nuances between Antibody-Positive and Seronegative MG is crucial for neurologists in formulating an effective management plan. Advances in diagnostic techniques and therapeutic interventions continue to improve the quality of life for individuals living with this challenging condition, emphasizing the importance of personalized medicine in the realm of neurology.
Conclusion
In conclusion, Myasthenia Gravis (MG), whether antibody-positive or seronegative, presents a complex clinical challenge that demands a nuanced approach to diagnosis and treatment. The differentiation between antibody-positive and seronegative MG underscores the importance of precise diagnostic techniques and the need for ongoing research to better understand and address the underlying immune mechanisms. Despite the challenges in diagnosing seronegative MG due to the absence of specific antibodies, advancements in clinical evaluation and diagnostic technologies continue to improve the accuracy of diagnosis, enabling tailored treatment strategies. Treatment for both types of MG focuses on symptom management, immune modulation, and, when necessary, surgical interventions, with the goal of enhancing patient quality of life and minimizing the impact of the disease on daily activities. The journey of understanding and managing MG illustrates the critical role of personalized medicine in neurology, highlighting the necessity for individualized care plans that address the unique presentation and treatment response of each patient.

Dr. Kashouty, a diplomate of the American Board of Psychiatry and Neurology (ABPN), practices general neurology with fellowship trained specialization in clinical neurophysiology. Dr. Kashouty finds the form and function of the nerves and muscles the most interesting part of neurology, which is what led him to specialize in neurophysiology with more emphasis on neuromuscular conditions. He treats all neurological diseases, but his main focus is to treat and manage headaches, movement disorders and neuromuscular diseases.




